Research
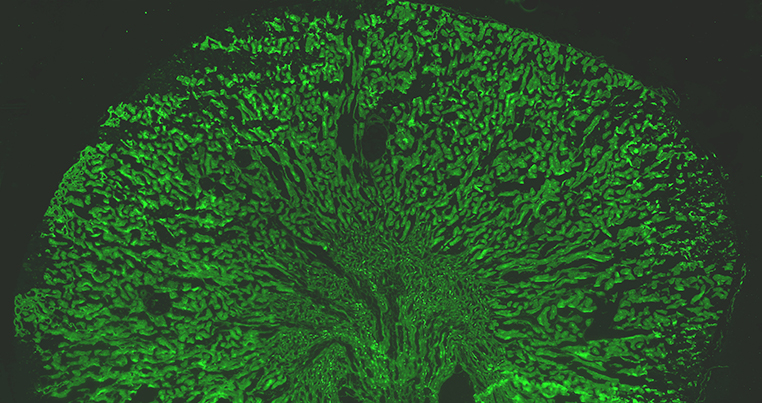
Expression of the V-ATPase in mouse kidney
Research Interests of the KRC
KRC’s research interests are driven by clinical needs. We are interested in finding the causes of, better cures and treatments for all kidney diseases through basic, translational, and clinical research.
Currently, we have research projects in the following six areas of kidney diseases:
Acute kidney injury (AKI) is defined as an abrupt or rapid decline in renal filtration function. It can happen within a few hours or few days. The most common cause of acute kidney injury (AKI) in hospitalized patients is sepsis. However, the molecular pathways and mechanisms that mediate septic AKI are not well defined with the exception that experiments performed over the past 20 years have started to shed light on how sepsis modulates tubular transport function. One of our research areas is to use animal models as well as clinical data to study the links between septic inflammation and tubular transport dysfunction with impaired GFR and kidney failure. For example, there has been a renewed interest over the past 5 years in the role of chloride in septic AKI. Recent large clinical trials have shown that an increased serum chloride concentration may have a deleterious effect on overall kidney function, especially in septic patients. Tubular transport would be directly involved in these deleterious effects as septic AKI is mediated in part by tubular processes that increase chloride delivery to the distal tubule, and we suggest that this phenomenon may play a key role in overall organ dysfunction. We will also study the inflammatory pathways in septic AKI to identify where patients lie on the immunologic spectrum to appropriately target therapies at the inflammatory cascade, TLRs, and possibly apoptosis.
Hypertension is the leading cause of stroke and cardiovascular diseases, affecting a huge percentage of the adult population. High blood pressure is the number one global burden of disease risk factor. One of the main research focuses of KRC is to investigate the link between hypertension and the regulation of kidney sodium transport, to elucidate the renal mechanisms responsible for regulation of blood pressure, sodium and potassium balance, how homeostasis is disrupted in disease states and corrected therapeutically. We are studying the ion transporters’ phosphorylation, abundance, subcellular distribution and activity, and intending to define how stimuli such as dietary sodium and potassium, angiotensin II, injury, and cytokines increase renal sodium transport and how the resultant hypertension provokes intrarenal responses to decrease sodium reabsorption (via pressure natriuresis) and finding more individualized cures for hypertension in patients with kidney disease.
>Polycystic kidney disease (PKD) is an inherited condition defined by the pathological development of fluid-filled cysts throughout the kidneys leading to organ enlargement and chronic kidney disease. Autosomal dominant polycystic kidney disease (ADPKD) is the commonest type of PKD. It leads to end-stage renal disease by age 60 in ~50% of cases. To date, there is no proven preventive regime nor effective treatment available for ADPKD patients even though the genetic aspects of the disease are well understood. One of the major research projects in KRC is to study the functions of the proteins PC1 and PC2, which are the products of the two genes (pkd1 and pkd2) that cause ADPKD. We will also test the feasibility of AMPK as a therapeutic target in ADPKD, search for new therapies such as the antidiabetic drug metformin might slow the progression of ADPKD, and to explore the role of new metabolomics biomarkers in assessing the severity of the disease and its response to therapies.
Acid regulation is critical for normal physiology, metabolism, and cellular function. Two components in the plasma, CO2 that is regulated by alveolar ventilation after production in peripheral tissues and HCO3- that is predominantly regulated by renal acid-base handling. The kidneys reabsorb, produce, and in some circumstances, excrete HCO-. The kidneys have the predominant role of regulating the systemic HCO3- concentration and hence, the metabolic component of acid-base balance. We will study how the accumulation of acids in the blood of patients with kidney disease can worsen the progression of those ailments.
Currently, >10% of the US population is afflicted with CKD, a condition that is often asymptomatic. As the 9th leading cause of death in the US, CKD is defined as a significant reduction in kidney function (GFR) and/or kidney damage lasting for more than 3 months. Importantly, one in every two adults will develop CKD if they live to be 85 years old. CKD can result from many different kidney insults; e.g., damage caused by diseases like diabetes (DM) and hypertension. People living with CKD have an accelerated mortality and progression towards End Stage Kidney Disease (ESKD). Moreover, patients with CKD are also at risk for acute kidney injury (AKI). This condition is defined as a rapid decrease in GFR within hours or days and is common in hospitalized patients with sepsis and shock. AKI significantly contributes to morbidity, mortality and CKD progression. To survive with ESKD, patients require renal replacement therapy (RRT), dialysis or kidney transplantation. Dialysis mortality is equivalent to that of metastatic lung cancer (~20% per year). In contrast, a living donor kidney transplant offers a >90% five-year survival rate (vs. 81% for deceased donor transplants). However, there is a critical shortage of kidneys for transplantation, as 13 people die daily awaiting a transplant. At the KRC, we believe that it is important to investigate kidney regeneration in CKD, especially regarding druggable targets. Findings from our planned studies have the potential to improve kidney outcomes and are thus highly significant for public health. Moreover, by investigating how to slow CKD progression following nephron loss, we may improve kidney outcomes in the ~25% of living donors that develop CKD markers at one year after kidney donation.
Kidney cancer is a disease with high mortality. Renal cell carcinoma (RCC) is the most common type of kidney cancer. Patients with end stage kidney disease (ESRD) are at high risk for this malignancy. One protein, the vacuolar H+-ATPase (V-ATPase) pump plays an important role in the malignant potential of RCC. There is abnormally high expression of the enzyme Aurora kinase. This enzyme increases V-ATPase activity and may thus increase the potential for metastases in RCC. We will use special immunological tools to verify the diagnostic potential of in house developed antibodies for metastatic RCC in animal models as well as patients’ specimens in the hopes of finding new biomarkers that can predict the metastatic potential of RCC. We will also test whether activating AMP activated protein kinase (AMPK), a known inhibitor of the V-ATPase, such as antidiabetic drugs can decrease the metastatic potential of RCC cells.
Research Funding
KRC is jointly supported by USC Keck School of Medicine, Department of Medicine, Division of Nephrology and Hypertension, and the University Kidney Research Organization (UKRO).
Our Research Funding are partially from these two organizations as well as grants from government and foundation sources such as the N.I.H., the U.S. Department of Defense and the USC Wright Foundation.